Соединения металлов, в том числе соединения редкоземельных металлов, играют решающую роль в поглощении инфракрасного излучения. Являясь лидером в производстве редких металлов и редкоземельных соединений, УрбанМайнс Тех. Компания, ООО обслуживает почти 1/8 мировых клиентов, занимающихся поглощением инфракрасного излучения. Чтобы ответить на технические запросы наших клиентов по этому вопросу, центр исследований и разработок нашей компании составил эту статью, чтобы дать ответы.
1.Принцип и особенности поглощения инфракрасного излучения соединениями металлов.
Принцип поглощения инфракрасного излучения соединениями металлов основан главным образом на колебаниях их молекулярной структуры и химических связей. Инфракрасная спектроскопия изучает молекулярную структуру путем измерения перехода внутримолекулярных уровней вибрации и вращательной энергии. Вибрация химических связей в соединениях металлов приведет к поглощению инфракрасного излучения, особенно металл-органических связей в металлоорганических соединениях, вибрации многих неорганических связей и вибрации кристаллического каркаса, которые будут проявляться в различных областях инфракрасного спектра.
Характеристики соединений различных металлов в инфракрасных спектрах:
(1).Материал MXene: MXene представляет собой двумерное соединение переходного металла-углерода и азота с богатым содержанием компонентов, металлической проводимостью, большой удельной площадью поверхности и активной поверхностью. Он имеет различную степень поглощения инфракрасного излучения в ближнем инфракрасном и среднем/дальнем инфракрасном диапазонах и в последние годы широко используется в инфракрасном камуфляже, фототермическом преобразовании и других областях.
(2).Соединения меди: Фосфорсодержащие соединения меди хорошо работают среди поглотителей инфракрасного излучения, эффективно предотвращая явление почернения, вызванное ультрафиолетовыми лучами, и стабильно сохраняя превосходные свойства пропускания видимого света и поглощения инфракрасного излучения в течение длительного времени.
Практические случаи применения
(1). «Инфракрасный камуфляж»: материалы MXene широко используются в инфракрасном камуфляже благодаря их превосходным свойствам поглощения инфракрасного излучения. Они могут эффективно уменьшить инфракрасные характеристики цели и улучшить маскировку.
(2). «Фототермическое преобразование»: материалы MXene обладают низкими характеристиками излучения в среднем и дальнем инфракрасном диапазонах, которые подходят для применений фототермического преобразования и могут эффективно преобразовывать световую энергию в тепловую энергию.
(3).Оконные материалы. Композиции смол, содержащие поглотители инфракрасного излучения, используются в оконных материалах для эффективного блокирования инфракрасных лучей и повышения энергоэффективности.
Эти случаи применения демонстрируют разнообразие и практичность соединений металлов в поглощении инфракрасного излучения, особенно их важную роль в современной науке и промышленности.
2.Соединения каких металлов способны поглощать инфракрасные лучи?
Соединения металлов, способные поглощать инфракрасные лучи, включают оксид сурьмы и олова (ATO), оксид индия и олова (ITO), оксид алюминия-цинка (AZO), триоксид вольфрама (WO3), четырехокись железа (Fe3O4) и титанат стронция (SrTiO3).
2.1 Характеристики инфракрасного поглощения соединений металлов
Оксид сурьмы и олова (ATO): он может экранировать ближний инфракрасный свет с длиной волны более 1500 нм, но не может экранировать ультрафиолетовый и инфракрасный свет с длиной волны менее 1500 нм.
Оксид индия и олова (ITO): Подобно ATO, он имеет эффект экранирования ближнего инфракрасного света.
Оксид цинка и алюминия (AZO): он также выполняет функцию экранирования ближнего инфракрасного света.
Триоксид вольфрама (WO3): Он обладает эффектом локализованного поверхностного плазмонного резонанса и небольшим механизмом поглощения поляронов, может экранировать инфракрасное излучение с длиной волны 780-2500 нм, нетоксичен и недорог.
Fe3O4: он обладает хорошими свойствами поглощения инфракрасного излучения и термического отклика и часто используется в инфракрасных датчиках и детекторах.
«Титанат стронция (SrTiO3): обладает отличным поглощением инфракрасного излучения и оптическими свойствами, подходит для инфракрасных датчиков и детекторов».
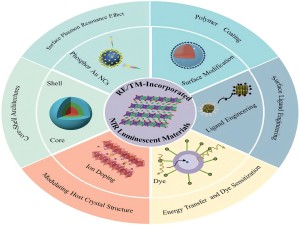
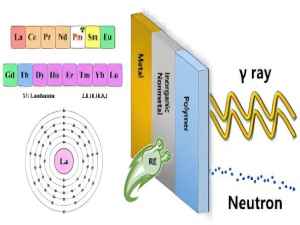
Фторид эрбия (ErF3): редкоземельное соединение, способное поглощать инфракрасные лучи. Фторид эрбия имеет кристаллы розового цвета, температуру плавления 1350°С, температуру кипения 2200°С и плотность 7,814 г/см³. Он в основном используется в оптических покрытиях, легировании волокон, лазерных кристаллах, монокристаллическом сырье, лазерных усилителях, добавках к катализаторам и других областях.
2.2 Применение соединений металлов в материалах, поглощающих инфракрасное излучение
Эти соединения металлов широко используются в материалах, поглощающих инфракрасное излучение. Например, ATO, ITO и AZO часто используются в прозрачных проводящих, антистатических, радиационно-защитных покрытиях и прозрачных электродах; WO3 широко используется в различных теплоизоляционных, поглощающих и отражающих инфракрасных материалах благодаря своим превосходным характеристикам экранирования ближнего инфракрасного диапазона и нетоксичным свойствам. Эти соединения металлов играют важную роль в области инфракрасных технологий благодаря своим уникальным характеристикам поглощения инфракрасного излучения.
2.3. Какие редкоземельные соединения способны поглощать инфракрасные лучи?
Среди редкоземельных элементов гексаборид лантана и наноразмерный борид лантана способны поглощать инфракрасные лучи. Гексаборид лантана (LaB6) - это материал, широко используемый в радиолокационной, аэрокосмической, электронной промышленности, приборостроении, медицинском оборудовании, металлургии бытовой техники, защите окружающей среды и других областях. В частности, монокристалл гексаборида лантана является материалом для изготовления мощных электронных ламп, магнетронов, электронных и ионных пучков, катодов ускорителей.
Кроме того, наноразмерный борид лантана также обладает свойством поглощать инфракрасные лучи. Он используется в покрытии поверхности листов полиэтиленовой пленки для блокировки инфракрасных лучей солнечного света. Поглощая инфракрасные лучи, наноразмерный борид лантана не поглощает слишком много видимого света. Этот материал может предотвратить попадание инфракрасных лучей в оконное стекло в жарком климате и более эффективно использовать световую и тепловую энергию в холодном климате.
Редкоземельные элементы широко используются во многих областях, включая военную промышленность, атомную энергетику, высокие технологии и товары повседневного спроса. Например, лантан используется для улучшения тактических характеристик сплавов в вооружении и технике, гадолиний и его изотопы используются в качестве поглотителей нейтронов в области ядерной энергетики, а церий используется в качестве добавки к стеклу для поглощения ультрафиолетовых и инфракрасных лучей.
Церий как добавка к стеклу может поглощать ультрафиолетовые и инфракрасные лучи и в настоящее время широко используется в автомобильных стеклах. Он не только защищает от ультрафиолетовых лучей, но и снижает температуру внутри автомобиля, тем самым экономя электроэнергию на кондиционирование. С 1997 года в японские автомобильные стекла добавляют оксид церия, а с 1996 года он стал использоваться в автомобилях.
3.Свойства и факторы, влияющие на поглощение инфракрасного излучения соединениями металлов.
3.1 Свойства и факторы, влияющие на поглощение инфракрасного излучения соединениями металлов, в основном включают следующие аспекты:
Диапазон скорости поглощения: Скорость поглощения соединений металлов инфракрасных лучей варьируется в зависимости от таких факторов, как тип металла, состояние поверхности, температура и длина волны инфракрасных лучей. Обычные металлы, такие как алюминий, медь и железо, обычно имеют степень поглощения инфракрасных лучей от 10% до 50% при комнатной температуре. Например, степень поглощения инфракрасных лучей поверхностью чистого алюминия при комнатной температуре составляет около 12%, тогда как степень поглощения шероховатой медной поверхности может достигать около 40%.
3.2 Свойства и факторы, влияющие на поглощение инфракрасного излучения соединениями металлов:
«Типы металлов»: разные металлы имеют разную атомную структуру и расположение электронов, что приводит к разной способности поглощать инфракрасные лучи.
«Состояние поверхности»: шероховатость, оксидный слой или покрытие металлической поверхности влияют на скорость поглощения.
«Температура»: изменения температуры изменят электронное состояние внутри металла, тем самым влияя на его поглощение инфракрасных лучей.
«Инфракрасная длина волны»: разные длины волн инфракрасных лучей обладают разной способностью поглощать металлы.
«Изменения при определенных условиях»: при определенных условиях скорость поглощения инфракрасных лучей металлами может значительно измениться. Например, если металлическую поверхность покрыть слоем специального материала, ее способность поглощать инфракрасные лучи может быть повышена. Кроме того, к увеличению скорости поглощения могут приводить и изменения электронного состояния металлов в высокотемпературных средах.
«Области применения»: свойства соединений металлов по поглощению инфракрасного излучения имеют важное прикладное значение в инфракрасных технологиях, тепловидении и других областях. Например, контролируя покрытие или температуру металлической поверхности, можно регулировать ее поглощение инфракрасных лучей, что позволяет применять ее для измерения температуры, тепловидения и т. д.
«Экспериментальные методы и предпосылки исследования»: Исследователи определили скорость поглощения инфракрасных лучей металлами посредством экспериментальных измерений и профессиональных исследований. Эти данные важны для понимания оптических свойств соединений металлов и разработки соответствующих приложений.
Таким образом, на свойства соединений металлов по поглощению инфракрасного излучения влияют многие факторы, и они могут существенно меняться в различных условиях. Эти свойства широко используются во многих областях.















 IPv6 ПОДДЕРЖИВАЕМАЯ СЕТЬ
IPv6 ПОДДЕРЖИВАЕМАЯ СЕТЬ
